
CLINICALLY VALIDATED APPROACH
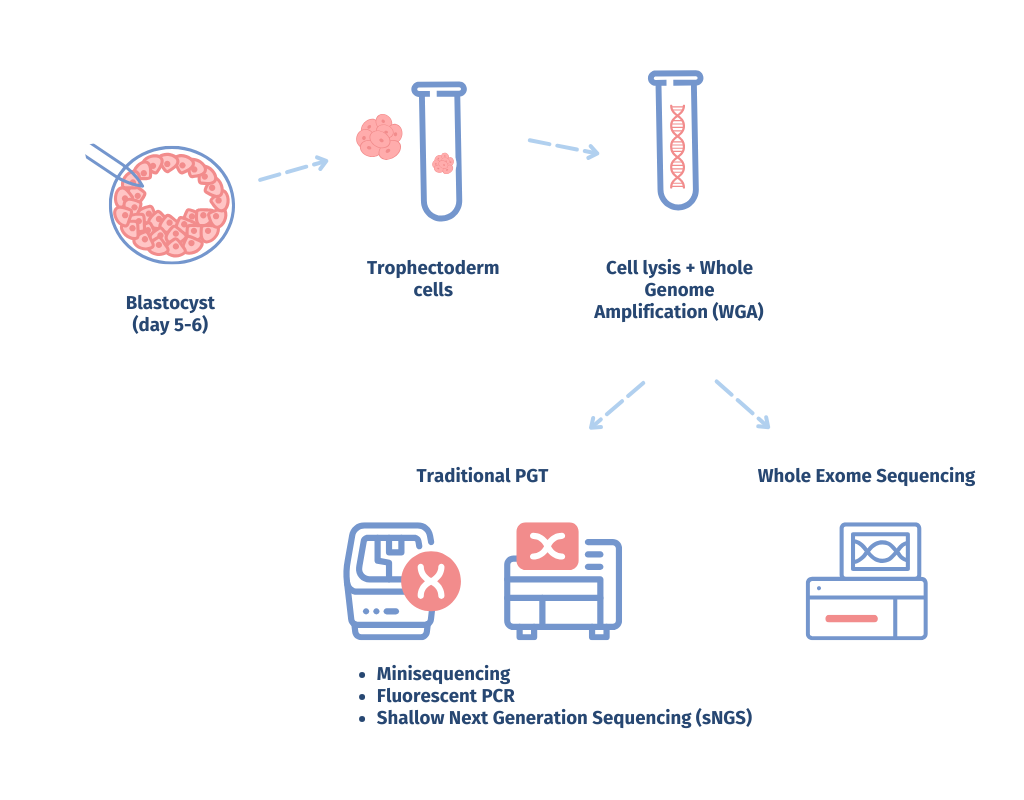
GENOMICA's researchers conducted an extensive validation for whole exome sequencing-based PGT (PGT-WES). The validation included a prospective, double-blind study comparing PGT-WES with conventional PGT methods. EmbryoGenome has demonstrated high accuracy in detecting inherited and de novo pathogenic variants, aneuploidy, and microdeletion/microduplication syndromes on standard trophectoderm biopsies of preimplantation embryos, providing unparalleled insights into embryo genetics.
Inherited genetic disorders
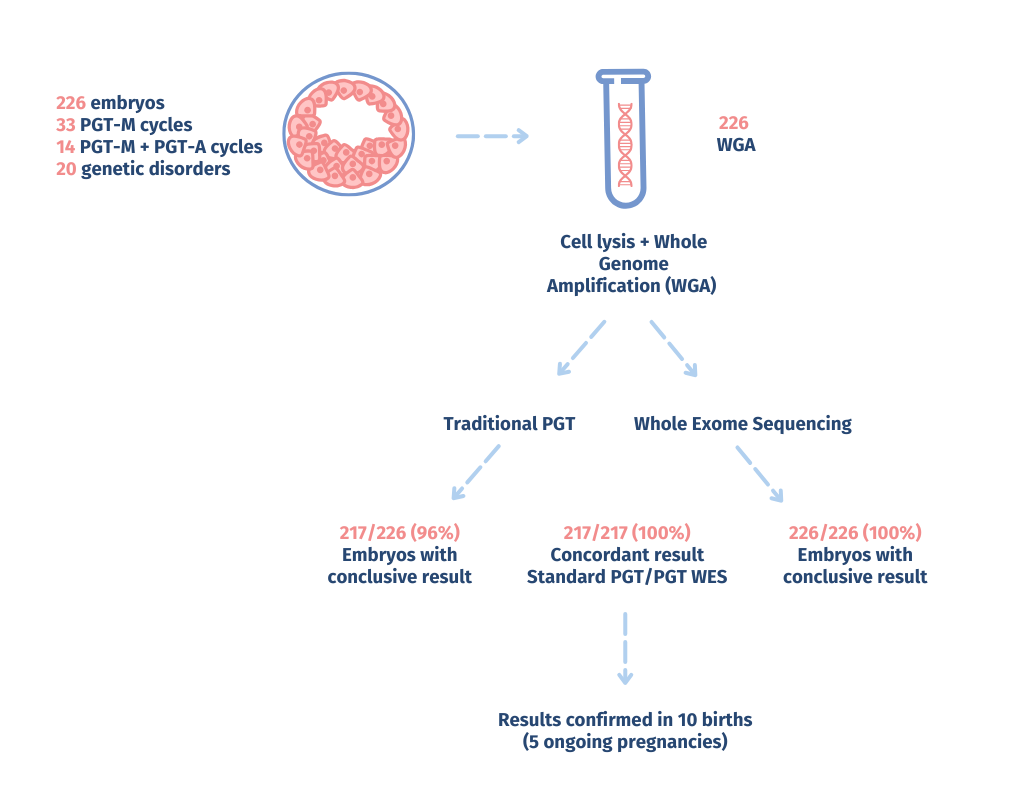
The validation results showed a high level of concordance with standard PGT methods. Outcomes for PGT-M were fully consistent across all embryos tested, reliably detecting target mutations and uncovering additional genetic variations associated with monogenic disorders—even in embryos from unrecognized carrier parents.
Malattie genetiche ad insorgenza de novo
EmbryoGenome accurately detected de novo pathogenic mutations linked to severe early-onset genetic conditions.
Chromosomal abnormalities
The CNV detection capability of EmbryoGenome matched the performance of standalone PGT-A assays, accurately identifying both whole-chromosome and segmental aneuploidies. Ploidy status was 100% concordant across all embryos screened for PGT-A. Pathogenic CNVs >200 kb associated with parental microdeletion/microduplication syndromes were reliably detected, owing to the enhanced resolution of PGT-WES.
PGT-WES
- 5 pathogenic de novo mutations
- 8 embryos affected by inherited disorders caused by mutations from unaware carrier parents
- 9 embryos, for which an inconclusive result was obtained with the standard PGT analysis, a conclusive result was obtained with PGT-WES
- pathogenic /strong>CNVs >200 Kb associated with microdeletion / microduplication syndromes were identified


 Italiano
Italiano English
English